Leeds Sweet RCT
The LCH Library and Research teams worked together to run the Leeds Sweet RCT (a mock randomised trial) at the Trust's 2025 Clinical Conference, and also at an Adult Business Unit Celebration event later in the year.
The aim was to have a fun activity that drew delegates to our stall, helped raise awareness of research and started conversations with colleagues about how they could link with the library and research teams.
The idea for the trial was based on the Randomised Chocolate Trial carried out and kindly shared by the Library Team at Wirral University Hospital Trust in pre-pandemic times.
This page gives an overview of the methodology used (the participant information and research protocol), an example of the MS Form used during the trial, and also the write-up of trial.
Thank you for your interest in this piece of work. For any queries, please contact either the LCH Library Team or the LCH Research Team.
Participant information
Title of Study: A randomised confectionery trial to compare the effects of eating sherbet lemons or chocolate eclairs on the wellbeing of staff in the Leeds health and care economy.
Introduction
We would like to invite you to take part in a research study about eating different confectioneries. Before you decide whether to take part, it is important that you understand why the research is being done and what it involves. Members of our team will be available to answer any questions you may have. Feel free to also talk to your friends and colleagues about the study, if you wish. The study protocol has been included in case you wish to see it (see below). Please ask us if you would like more information.
What is the purpose of the study?
The study is trying to find out if eating sherbet lemons or chocolate eclairs have different effects on staff wellbeing.
Do I have to take part?
Taking part in this study is entirely voluntary. If you decide you wish to take part you will be asked to complete a Consent Form before proceeding. If you decide that you don’t wish to take part in this study you do not need to do anything further and your day will continue as normal.
What will happen if I do take part?
If you do decide to take part in this study your day will continue as usual; the only difference will be you will receive a sherbet lemon or chocolate éclair (selected at random), and after eating your selected confectionery you will be asked to complete a short questionnaire about your experience.
What are the benefits of taking part?
By taking part in this study, you will gain experience of being a participant in a randomised controlled trial.
You will also be helping the researchers to gain a greater understanding of whether eating sherbet lemons or chocolate eclairs improves staff wellbeing.
Will my details be confidential?
Yes. We will follow all data protection laws and all information about you will be kept strictly confidential.
What will happen to the results of the study?
When the study is complete the results will be presented in the LCH Trust Communications Bulletin, on the Research Department pages of the LCH intranet and on the Library web pages.
Who is organising and funding the study?
The study is being organised and funded by the Research Department and Library Service at Leeds Community Healthcare NHS Trust.
Thank you for taking the time to read this information.
*** To take part in the study whist at the conference, please follow the link here on a phone / mobile device ***
Research Protocol - Leeds Sweet RCT (ABU event)
Full Research Title: A randomised confectionery trial to compare the effects of eating sherbet lemons or chocolate eclairs on the wellbeing of staff in the Leeds health and care economy.
Short Title: Leeds Sweet RCT
Chief Investigator: Christine Comer, Clinical Research Fellow
Sub Investigator: Helen Swales, Library Services Manager
Background:
There is some evidence that consuming confectioneries can offer a short-term boost to wellbeing by providing a temporary source of comfort and pleasure. This is primarily due to the psychological effects of sugar, which can alter brain chemistry and temporarily reduce stress. Anecdotal evidence reported by members of the Research and Library teams confirms short term improvements in wellbeing, but it is unclear whether chocolate has a greater or smaller effect than other confectioneries.
Primary Research Question: Does eating sherbet lemons or chocolate eclairs affect wellbeing of staff in the Leeds health and care economy in the short-term?
Secondary Question: Is there a difference in the effects of different confectioneries on short-term wellbeing?
Inclusion Criteria:
- Member of staff in the Leeds health and care economy
- Willingness to eat chocolate or other confectionery
- Willingness to complete short questionnaire
- Able to give informed consent
- Able to access the questionnaires via phone or other digital device
Exclusion Criteria:
- Allergy to ingredients in sherbet lemons or chocolate eclairs
- Intolerance to ingredients in sherbet lemons or chocolate eclairs
- Other medical reason for not ingesting ingredients in sherbet lemons or chocolate eclairs
Recruitment Aim: 50
Methodology
This research will take place over one day: Tuesday 30th September. This date has been chosen to coincide with the LCH ABU Celebration event.
Members of staff in the Leeds health and care economy will be invited to take part in the trial during refreshment breaks on the day. Potential participants will be invited in person as they visit the research and library conference stands.
Potential participants will be provided with a digital Participant Information Sheet which they will be able to access on their smartphone via a QR code. There will be an opportunity to ask questions about the research. Once potential participants have had all their questions answered they will complete a digital eligibility checklist. If they confirm that they wish to participate and meet all the inclusion criteria and none of the exclusion criteria, they will be able to proceed to the consent form.
After completion of the digital consent form, participants will then be randomised using a “lucky dip” randomised technique, to receive either the sherbet lemon intervention or the chocolate eclair intervention. Once the participant has eaten their confectionery, they will be asked to complete a short digital questionnaire about their experience which will be platformed on an Microsoft Teams Survey Form.
Data will be analysed within the Microsoft survey form. If more detailed analysis is warranted and appropriate, data will be exported to an Excel spreadsheet. Results will be published via Trust Communications Bulletin and Research Department and Library Services web pages.
Sample form
We used MS Forms to manage the trial on the day and to collect the data.
A sample of the form is shown here.
If you would like a copy of the form, please contact helen.swales@nhs.net
Write up
LEEDS SWEET TRIAL: a mock randomised confectionery trial to evaluate the effects of eating sherbet lemons or chocolate eclairs on the wellbeing of staff in the Leeds health and care system
Helen Swales, Christine Comer, Leeds Community Healthcare NHS Trust
ABSTRACT
Our Leeds Sweet Trial resulted in a positive immediate impact on wellbeing for the majority of participants in the trial (80%). Interestingly, participants at two different events reported similar positive effects after eating chocolate eclairs, but very different effects on wellbeing after eating sherbet lemon sweets. While taking part, participants engaged in conversations about research with members of the library and research teams. This fun activity was a great way to raise awareness of research and library department activities.
INTRODUCTION
In June 2025, Leeds Community Healthcare NHS Trust (LCH Trust) held a mock randomised trial, the Leeds Sweet RCT, for LCH Trust and GP confederation staff. The aim was to raise awareness of clinical research and the work of LCH Trust library services and research department. As this was well-received, we repeated the exercise at a celebration event for the LCH Adult Business Unit in September 2025.
Research activity needs to grow in community and primary care settings across Leeds to help tackle the challenging shift from hospital care to community care outlined in the NHS 10-year Plan (1). Developing research knowledge, experience and confidence among our staff is crucial and can be enabled through opportunities to engage in a range of research activities, supported by our research and library teams.
Increasing research activity not only leads to the delivery of more effective and efficient healthcare (2) but also improves staff engagement and morale through improved job satisfaction (3). Maintaining wellbeing of NHS staff is of paramount importance (1). Whilst research activity offers staff development opportunities (4) which might contribute to long-term wellbeing, anecdotal evidence from researchers and librarians suggests that sweet confectioneries might be another way to improve how staff feel in the short term.
To engage staff in a research discussion and activity, we ran a mock randomised trial comparing responses to two different types of sweet. The aims of the trial were
-
to investigate whether staff wellbeing improved immediately after being given a chocolate éclair or sherbet lemon sweet to eat
-
to improve knowledge and understanding of clinical trials in a fun way and to engage staff in conversations about research
METHODS
We invited all delegates who approached our research and library information stand at a clinical conference event in June 2025 and at a celebration event (Adult Business Unit) in September 2025 to take part in the trial. At both events, the research and library stand was set up in a small room, alongside multiple other stands available for delegates to visit during refreshment and lunch breaks throughout the conference/ event.
We recruited participants to the trial and obtained their informed consent – participants were asked to read the study information leaflet, to ask any questions, to confirm that they were eligible and that there were no reasons for them not to participate, and then to tick a box on the digital form to confirm that they were happy to take part (consent).
The two confectionery interventions were both wrapped sweets: Chocolate eclairs are chewy caramel sweets with a milk chocolate centre, and Sherbet lemons are hard, lemon-flavored candies with a fizzy, sherbet centre.
Participants were randomised to one of the two interventions by selecting a single sweet at random from a basket using a pair of tongs. The basket was held above eye-line height. This meant that participants and researchers were blinded until the sweet was picked out of the basket, so that they were not able to influence the selection. There was no blinding of the intervention beyond this point, meaning that both participants and researchers were aware of which sweet had been selected.
After completing the trial intervention by eating their sweet, we asked participants to report the outcome of the intervention by responding on the digital form to a question about how their wellbeing had been affected by eating the sweet.
Data were captured using Microsoft Forms and exported directly to Excel for analysis. The analysis involved using descriptive statistics, including frequency data for participant information and the categorial outcome data. Given the small sample size and lack of data in specific response categories, interferential statistical analyses of the outcome data using Chi-Square (χ2) tests were not appropriate.
RESULTS
Participation
Delegates at the conference and at the celebration event were drawn to our stand as lots of activity and fun was generated while recruiting to the trial. Of 78 potential participants visiting the research stand who were approached with information about the trial and invited to take part, 76 were recruited and participated in the trial. Two did not proceed to informed consent because of ineligibility (not employed by a Leeds Health or care organisation n = 1; unable to/ should not eat the confectionery included in the trial n = 1).
At the clinical conference, half the participants (n = 18) let us know that they work for Leeds Community Healthcare Trust, with the other half identifying their place of work as Primary Care in Leeds (n = 1), ‘Other’ (n = 3) or no response (n = 15). At the celebration event, all participants were employed by Leeds Community Healthcare Trust and working in the Adult Business Unit or corporate teams.
Effects of consuming confectionery on wellbeing
Of the 76 participants in the trial, 34 (34/76 = 45%) randomly selected a chocolate éclair, and 42 (55%) randomly selected a lemon sherbet.
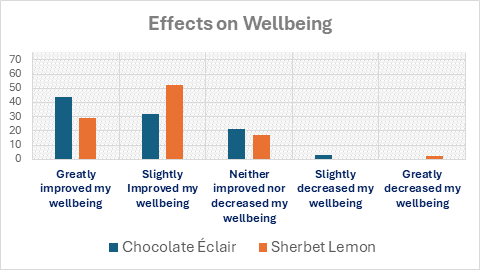
After consuming their randomly selected confectionery, 61 of 76 (80%) participants reported an improvement in wellbeing (slightly improved wellbeing n=33 (43%); greatly improved wellbeing n=28 (37%)). One participant reported slightly decreased wellbeing and another reported greatly decreased wellbeing, whilst 14 (18%) reported neither improved nor decreased wellbeing following consumption.
The participant-reported effects on wellbeing of the different confectionery types are shown in Figure 1, which can be seen here and also below the references.
Comparing Chocolate Eclair and Sherbet Lemon outcomes
A slightly higher proportion (81%) of those who selected a sherbet lemon reported improvements in wellbeing (slightly improved or greatly improved) compared to those who selected a chocolate éclair (76%). Wellbeing decreased in two participants (3%), one reporting a great decrease in wellbeing after consuming a sherbet lemon and the other reporting a slight decrease in wellbeing after consuming a chocolate éclair. For each type of sweet, 7 participants (9%) reported no change in wellbeing.
Comparing results from participants at Clinical conference to participants at Adult Business Unit celebration event
Participant-reported improvements in wellbeing after eating chocolate eclairs was similar for participants at both events (74% and 79% participants at the clinical conference and adult business unit celebration event respectively). For sherbet lemons, a greater proportion of participants at the clinical conference reported benefits (95%, of which 52% greatly improved and 43% slightly improved) compared to those attending the adult business unit celebration (67%, of which 5% greatly improved and 62% slightly improved) and one participant at this event reported greatly decreased wellbeing following consumption of a sherbet lemon.
Table 1. Reported impact on wellbeing for participants at each event
| Clinical conference participants | Adult Business unit Celebration Event participants | ||
| Chocolate Éclair | Sherbet Lemon | Chocolate Éclair | Sherbet Lemon |
Greatly improved wellbeing | 47% | 52% | 42% | 5% |
Slightly Improved wellbeing | 27% | 43% | 37% | 62% |
Neither improved nor decreased | 20% | 5% | 21% | 28% |
Slightly decreased wellbeing | 6% | 0% | 0% | 0% |
Greatly decreased wellbeing | 0% | 0% | 0% | 5% |
Staff Engagement
Of the 76 participants, 34 (45%) said that they would like to be kept informed of news and updates from the LCH Trust research department and library service and provided their email address for this purpose. All those who took part had conversations with members of the research department team and/or library service team.
The 40 participants from the adult business unit celebration event in September were asked two additional questions about how participation in the mock trial had affected their enthusiasm for research and their understanding of how a research trial works. Responses are summarised in table 2.
Table 2. impact of participation on research enthusiasm and understanding
How did taking part affect enthusiasm for research? | ||
A lot | A little bit | Not at all |
5 (12.5%) | 30 (75%) | 5 (12.5%) |
How did taking part affect understanding of research trials? | ||
A lot | A little bit | Not at all |
9 (22.5%) | 27(67.5%) | 4 (10%) |
DISCUSSION
This study met its aims of investigating the effects of two different sweet confectioneries on staff wellbeing and engaging staff in conversations about research and the work of the research and library teams.
Our main finding was that sweet confectioneries had a positive short-term impact on wellbeing for the majority of participants in the trial (80%). Overall, the benefits following consumption of chocolate eclairs (76% improved wellbeing) and sherbet lemons (81% improved wellbeing) were similar. However, participants at the clinical conference were much more likely to report great improvements in wellbeing after eating a sherbet lemon (52%) compared to participants from the Adult Business Unit celebration event (5%), with one participant at this event who selected a sherbet lemon even reporting greatly decreased wellbeing.
All those who took part at either event engaged in conversations about research or about the work of the research and library teams. Furthermore, responses from participants at the Adult Business Unit celebration event indicate that the majority of participants had increased enthusiasm for research (87.5% participants) and understanding of research trials (90% participants) after taking part.
These results need to be interpreted with some caution in light of some study limitations which may have biased our study findings: The small sample of self-selecting participants were drawn from delegates at two different events, the first of which took place on a very warm day, which may have contributed to the different participant responses at each event. At both events, the trial was conducted in small, crowded rooms with limited time for participants to read and digest the study information or to enjoy their sweet. Whilst selection of the sweets was blinded, both researchers and participants were unblinded during consumption and outcome data collection presenting a risk of biased responses.
This opportunity to participate in some real-life research, presented in a fun way, attracted delegates to start conversations with members of our research and library teams as well as resulting in some short-term improvements in wellbeing. Whilst this small study won't change the world, it proved to be a valuable marketing tool, attracting delegates to our stands at two separate events. Participants hopefully enjoyed the experience, had an opportunity to meet and engage with research and library team members who they will now recognise at future events, and this experience might encourage more staff to think about becoming involved in research activities in the future.
References
- NHS England, 2025. 10 Year Health Plan for England. Available at Fit for the future: 10 Year Health Plan for England (accessed on 29th August 2025).
- Jonker, L., Fisher, S.J. and Dagnan, D., 2020. Patients admitted to more research‐active hospitals have more confidence in staff and are better informed about their condition and medication: Results from a retrospective cross‐sectional study. Journal of evaluation in clinical practice, 26(1), pp.203-208.
- Marjanovic, S., Ball, S., Harshfield, A., Dimova, S., Prideaux, R., Carpenter, A., Punch, D. and Simmons, R., 2019. Involving NHS staff in research. The Healthcare Improvement Studies Institute. Available at Involving-NHS-Staff-In-Research-1.pdf (accessed 1st September 2025)
- Hanbury, A., Parker, E., Lawton, R., Marran, J., Schofield, J., Cave, L., McVey, L., Eyers, E., Van der Graaf, P. and Kislov, R., 2025. The benefits for health care staff of involvement in applied health research: a scoping review. Health Research Policy and Systems, 23(1), p.104.